In the intricate world of pharmaceuticals, every element, no matter how seemingly mundane, plays a pivotal role in ensuring product safety and efficacy. One such unsung hero is water. Water is not just a ubiquitous substance; it is a fundamental ingredient in pharmaceutical production, from being a component in medications to the equipment cleaning process. The control of water quality is an essential component of pharmaceutical operations. This blog post delves into the crucial realm of microbiological water testing, where the purity and safety of pharmaceutical water are meticulously safeguarded.
The Significance of Microbiological Water Testing
In the pharmaceutical industry, water's purity is paramount, as even trace microbial contamination can compromise product integrity and pose health risks to consumers. Microorganisms have a remarkable ability to thrive and proliferate in water, making it susceptible to contamination at various stages of its journey within pharmaceutical facilities. This journey includes entrance to the treatment plant, purification, storage, and distribution. The key to maintaining water purity lies in microbiological control at these critical stages.
Water Sampling: A Precise Art
The process begins with the collection of water samples, a meticulous task that requires attention to detail. Once a sample is collected, it must be stored at a temperature ranging between 2 and 10°C. This specific storage condition is necessary to preserve the integrity of the sample for analysis. The clock starts ticking upon collection - the microbial count must be performed within 8 hours, and the analysis of coliform bacteria must conclude within 30 hours. Stringent timelines are essential to maintain the accuracy and reliability of the results.
Classifying Pharmaceutical Water
Pharmaceutical water serves various purposes, and its classification depends on its intended use and the method of production. Here are the primary classifications:
- Water for Injections: This is used as a vehicle for bulk injection water or as a diluent for preparing parenteral medications.
- Purified Water: This water is used for medications that require sterility and apyrogenicity, except those where other water types are authorized.
- Water for Preparation of Extracts: It is specifically used for preparing medication extracts.
- Highly Purified Water: Reserved for medicinal products that demand a high biological quality, except in cases where water is used for injectables.
The Testing Process
Microbiological testing of pharmaceutical water is an exact science. It involves the use of a filter membrane with a pore size not exceeding 0.45 μm. The filtered microorganisms are then cultured, and the incubation is carried out at 30-35°C for a period of 5 days.
Here are the accepted microbial limits for different types of pharmaceutical water:
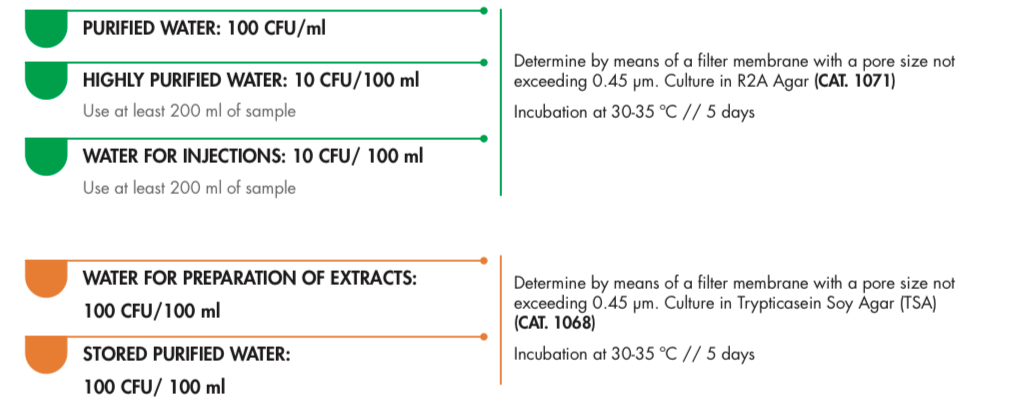
- Purified Water: 100 CFU/ml
- Highly Purified Water: 10 CFU/100 ml (using at least 200 ml of the sample)
- Water for Injections: 10 CFU/100 ml (using at least 200 ml of the sample)
- Water for Preparation of Extracts: 100 CFU/100 ml
- Stored Purified Water: 100 CFU/100 ml
The Guardians of Pharmaceutical Water Purity
Microbiological water testing is the unsung guardian of pharmaceutical water purity. It ensures that water, a seemingly simple component, meets the highest standards of quality and safety. As we navigate the intricacies of pharmaceutical production, the precision of microbiological water testing remains a steadfast ally, assuring the safety of every pharmaceutical product.
Elevate Your Microbial Testing with Condalab's Specialized Agar Media
In the quest for pharmaceutical safety and product quality, precision is the cornerstone. This precision extends to the tools and techniques employed in microbial testing, where the right agar media can make all the difference. Condalab, a trusted name in the field of microbiology, offers a range of specialized agar media that are not just reliable but can be the game-changer in your quality control processes.
Unveiling the Power of Agar Media: R2A Agar (CAT. 1071)
First on the list is the renowned R2A Agar, a versatile and highly effective medium for microbial analysis. This specialized agar is designed to enumerate microorganisms from water samples. With a filter membrane having a pore size not exceeding 0.45 μm, microorganisms are captured and cultured on R2A Agar. The incubation is carried out at a moderate temperature range of 30-35°C for a duration of 5 days.
Why R2A Agar Matters:
Versatility: R2A Agar is suitable for a wide range of applications, making it an invaluable tool in various industries, including pharmaceuticals, food production, and cosmetics.
Reliability: The selective components in R2A Agar enable it to foster the growth of diverse microorganisms, making it a comprehensive solution for microbial enumeration.
Accurate Results: The incubation process on R2A Agar ensures that you obtain precise and dependable microbial counts, vital for quality control.
The Precision of Trypticasein Soy Agar (TSA) (CAT. 1068)
Next in our arsenal of specialized agar media is Trypticasein Soy Agar, often abbreviated as TSA. This medium is designed to culture and enumerate microorganisms in a variety of samples. Like R2A Agar, the filtration is carried out using a membrane with a pore size not exceeding 0.45 μm, and the incubation takes place at a moderate temperature range of 30-35°C over a span of 5 days.
Why TSA Matters:
Broad Applicability: TSA is a versatile medium that finds utility in a range of industries, from pharmaceuticals to food production, where precise microbial testing is essential.
Selective and Comprehensive: TSA's formulation makes it selective for a wide range of microorganisms, ensuring that it captures and cultures the diverse microbial populations that might be present in your samples.
Accuracy and Reliability: The incubation process on TSA guarantees the accuracy of microbial counts, giving you data that you can trust for quality assurance.
Why Choose Condalab's Agar Media?
Condalab's specialized agar media, including R2A Agar and TSA, are trusted by industries worldwide for their precision, reliability, and versatility. These agar media are not just tools for microbial testing; they are gateways to the safety and quality of your products. Whether you're in pharmaceuticals, food production, cosmetics, or any other industry that demands rigorous quality control, Condalab's agar media can be your reliable partners.
Elevate your microbial testing to a new level of precision and trust with Condalab's specialized agar media. These tools are not just about counting microorganisms; they are about counting on quality and safety.
Learn More About Condalab's Specialized Agar Media and how they can transform your quality control processes.

A solution for
every need
We see our role as that of a legal partner responsible for constantly finding practical and pragmatic solutions that are adapted to our clients' needs.
References:
- American Public Health Association (1985) Standard Method for the Enumeration of Water and Wastewater.
- European Pharmacopeia 9.3.
In a world where patient safety and product quality are paramount, the role of microbiological water testing is invaluable. Stay tuned for more insights into the intricate world of pharmaceuticals and the tools that preserve its integrity. In the realm of pharmaceuticals, precision is the foundation of trust and safety.