The cosmetics industry thrives on precision and excellence. Every product that lands in the hands of consumers should not only be visually appealing but also completely safe to use. ISO 17516:2004 sets the standard for ensuring the quality and safety of cosmetic products, particularly in terms of microbial control. In this blog, we'll delve into how Condalab products are integral in helping the cosmetics industry meet these strict standards, specifically in the enumeration and detection of aerobic mesophilic bacteria.
ISO 17516:2004 - A Vital Benchmark
ISO 17516:2004 focuses on the microbiological quality of cosmetic products. It emphasizes the need for meticulous assessment to prevent any potential harm to the consumers' health. The standard specifies the procedures and guidelines that ensure cosmetics are free from harmful microbial contamination, thereby safeguarding users.
Total Mesophilic Aerobics - A Microbial Challenge
In the realm of cosmetics, an essential aspect of ISO 17516:2004 deals with the total mesophilic aerobics. These are microorganisms that flourish in the presence of oxygen and at a temperature range of 20°C to 45°C. Measuring the count of these microorganisms provides an estimate of the overall microflora within a cosmetic product. However, it doesn't identify the specific types of microorganisms.
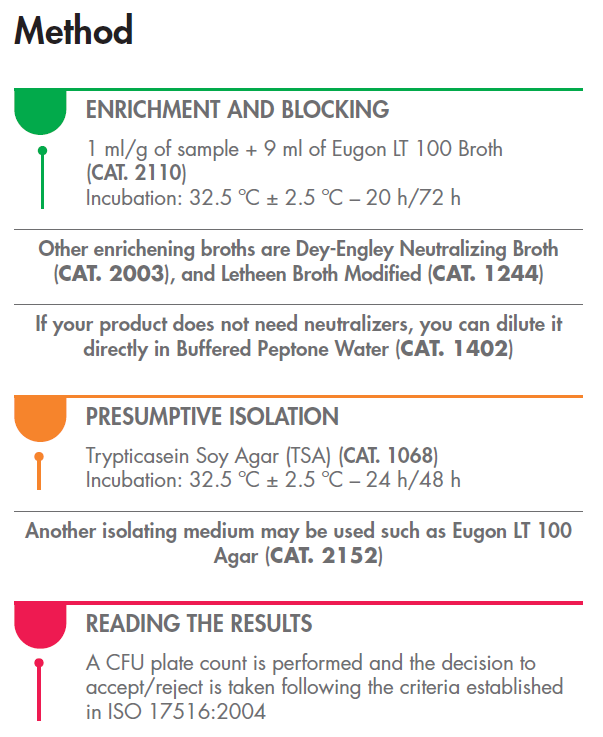
The Role of Condalab Products
Condalab offers a range of high-quality products crucial for the cosmetics industry to meet ISO 17516:2004. We understand that the total mesophilic aerobics can be a concern, and here's how we can assist:
1. Enrichment and Blocking: To initiate the process, our recommended product is the Eugon LT 100 Broth (CAT. 2110). A mixture of 1 ml/g of your sample with 9 ml of this broth can serve as an excellent starting point. The incubation time should be around 32.5°C ± 2.5°C for 20 to 72 hours.
Other enrichening broths are:- Dey-Engley Neutralizing Broth (CAT. 2003), and
- Letheen Broth Modified (CAT. 1244)
If your product does not need neutralizers, you can dilute it directly in
- Buffered Peptone Water (CAT. 1402)
2. Presumptive Isolation: For the next phase, we suggest Trypticasein Soy Agar (TSA) (CAT. 1068) for incubation. Like the previous step, the temperature should be maintained at 32.5°C ± 2.5°C for 24 to 48 hours.
Another isolating medium may be used such as Eugon LT 100 Agar (CAT. 2152)
3. Reading the Results: Once the incubation periods are complete, it's essential to perform a colony-forming unit (CFU) plate count. This count serves as the basis for accepting or rejecting products based on the criteria outlined in ISO 17516:2004.
Condalab's commitment to offering the cosmetics industry products that facilitate ISO compliance demonstrates our dedication to ensuring the highest standards of safety and quality for consumers. Our solutions help you effectively monitor and manage the total mesophilic aerobics, thus contributing to the overall quality assurance of cosmetic products.
Bibliography
- COLIPA. Guidelines on Microbial Quality Management, 1997 Published by the European Cosmetic, Toiletry and Perfumery Association (COLIPA).
- UNE-EN-ISO 21149:2017. Cosmetics. Microbiology. Enumeration and detection of aerobic mesophilic bacteria.
- E P. Microbiological Examination of non-sterile products, 4th edition, published by the European Pharmacopeaia, 2002.