In the intricate world of microbiology, yeasts and molds play a crucial role as members of the kingdom Fungi. This diverse group includes mushrooms, molds, and microscopic organisms such as yeasts. The identification and enumeration of these fungi are vital processes, particularly in industries like cosmetics where product safety and quality are paramount.
ISO 16212:2017, a standard set by the International Organization for Standardization, outlines a comprehensive procedure for the enumeration of yeasts and molds. This method is particularly relevant in the cosmetic industry, ensuring the microbial quality management of products.
Procedure Overview:
The procedure involves several key steps, each contributing to the accuracy and reliability of the results.
Enrichment and blocking are initiated by combining a 1ml/g of sample + 9ml of Eugon LT 100 Broth (2110) This broth is useful for the enrichment of aerobic bacteria present in cosmetics products. Incubation then takes place at 32.5 oC ± 2.5 oC – 20 h/72 h.
Other enriching broths are also available such as the Dey-Engley Neutralizing Broth (2003), used for the neutralization and testing of antiseptics and disinfectants commonly. There is also the Letheen Broth Modified (1244), a general use enrichment with neutralizers. In the case that a product does not need a neutralizer, it can be diluted directly in Buffered Peptone Water (1402).
Presumptive isolation, the subsequent step, involves Sabouraud Dextrose Agar with Chloramphenicol (1134) and requires precise incubation conditions. This agar is particularly useful in the selective cultivation and isolation of yeasts and molds. Incubation takes place at a temperature of 25 oC ± 2.5 oC – 3/5 days.
Lastly, reading the results involves a colony-forming unit (CFU) plate count, and the decision to accept or reject is made following the criteria established in ISO 17516:2014.:
In the dynamic field of cosmetic microbiology, adherence to standardized procedures is essential. The ISO 16212:2017 procedure provides a robust framework for the enumeration of yeasts and molds, ensuring the safety and quality of cosmetic products. By following these guidelines, industry professionals can confidently navigate the complex realm of microbial analysis, upholding the highest standards in cosmetic production.
Solutions for the Cosmetics Industry
While in this particular blog post, yeasts and molds are covered, there are several other pathogens that are of concern in the cosmetics industry. They are; Staphylococcus, E. Coli, Candida Albicans, and Pseudomonas Aeruginosa, these pathogens are mandatory to report in cosmetic products.
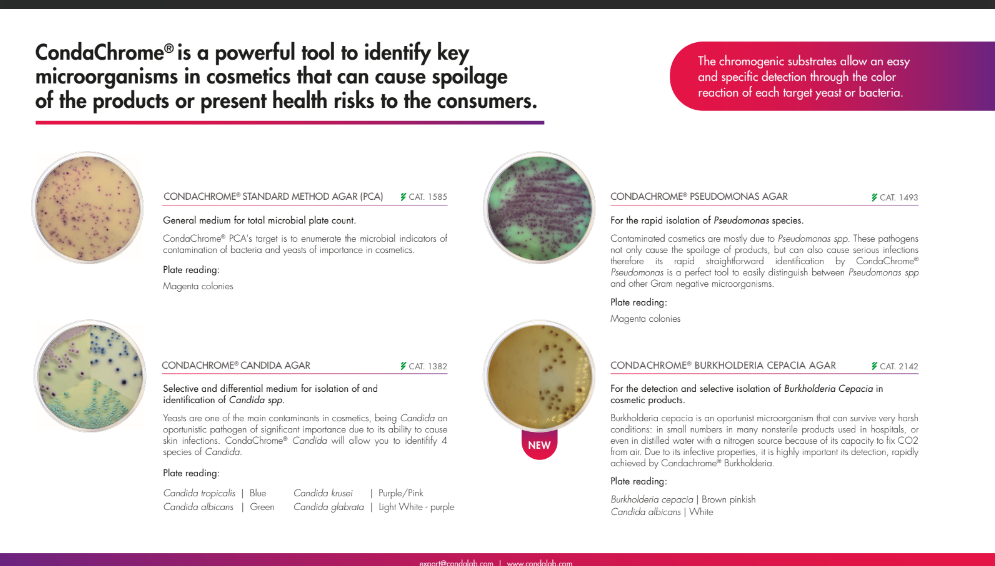
Condalab has recently developed two innovative chromogenic media that cover all four mandatory pathogens. Offered as a Condachrome Staphylococcus Agar (2076) and a Condachrome PEC Agar (2144), they are powerful tools to identify key microorganisms that can cause spoilage of the products or present health risks to consumers.
Sources:
1. COLIPA. Guidelines on Microbial Quality Management, 1997 Published by the European Cosmetic, Toiletry and Perfumery Association (COLIPA).
2. UNE-EN-ISO 16212:2017. Cosmetics. Microbiology. Enumeration of yeast and mould.
3. EN 13624:2003, Chemical disinfectants and antiseptics. Quantitative suspension test for the evaluation of fungicidal activity of chemical disinfectants for instruments used in the medical area. Test method and requirements (phase 2, step 1).